Oral sucrose administration to reduce pain response during immunization in 16-19-month infants: a randomized, placebo-controlled trial
AUTHOR/S
Gonca Yilmaz1, Nilgun Caylan2, Melek Oguz1 and Can Demir Karacan1
1Department of Social and Developmental Pediatrics, Dr. Sami Ulus Children and Maternity Training Hospital, Birlik Mah. Kahire Cad. 451 Sokak, Zirvekent Mimoza Sitesi A2 Blok, No 54, Çankaya, Ankara, Turkey
2Turkish Ministry of Health, Ankara, Turkey
Gonca Yilmaz (Corresponding author) Email: goncay31@gmail.com
Nilgun Caylan Email: ncaylan@hotmail.com
Melek Oguz Email: melekboynukalin@gmail.com
Can Demir Karacan Email: candecan@hotmail.com
Abstract
Although the analgesic effect of sucrose on newborns is well established, little is known about whether these solutions are effective in reducing procedural pain in infants beyond the newborn period. The purpose of this study was to determine the effect of sucrose solution given orally on infant crying times and measure the distress in a 16-19-month age group. A total of 537 healthy, 16-19-month-old infants attending for their immunizations with intramuscular diphtheria, tetanus, and acellular pertussis (DTaP)/Haemophilus influenza type b/IPV (along with oral polio vaccination (OPV)), intramuscular pneumococcus and intramuscular hepatitis A were randomized to receive 2 mL of a 75 % sucrose solution, a 25 % sucrose solution or sterile water 2 min before injections. Infants receiving a 75 % sucrose solution had significantly reduced total crying times and Children’s Hospital of Eastern Ontario Pain Scale scores (CHEOPS) compared with infants in the control and 25 % sucrose solution groups (p<0.001). Conclusion: Sucrose solution reduces infant distress and is safe and clinically useful even for 16-19-month-old infants.
Keywords: Analgesia - Infant - Immunization - Sucrose.
Abbreviations
Cheops - Children’s Hospital of Eastern Ontario Pain Scale
DTaP - Intramuscular diphtheria, tetanus, and acellular pertussis
Ipv - Inactive polio vaccination
Opv - Oral polio vaccination
Communicated by Beat Steinmann
Table of contents
Introduction
Return to the table of contents
Immunizations are among the most unpleasant medical procedures for healthy infants and children, and are the most common painful procedures of childhood. These procedures are known to be painful can result in anxiety, distress, and fear in children as well as their parents and the risk of longer-term fears of needle pain, parental nonadherence with vaccination administration, and avoidance of medical care [30, 32, 36]. The reduction of pain and distress during vaccinations has the potential to minimize subsequent fears of needles, needle phobia, noncompliance with scheduled immunizations, and later avoidance of medical care [33].
The administration of sweet solutions is now widely recommended for routine use during painful procedures in neonates [2, 3, 14, 31, 34]. Although the analgesic effect of sucrose on newborns is well established, little is known about whether these or other sweet tasting solutions effectively reduce procedural pain in infants beyond the newborn period [16, 19].
The aim of our study was to determine whether using 2 mL of a 75 % sucrose or a 25 % sucrose solution decreases the infant crying time and Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) scores after immunization with intramuscular diphtheria, tetanus, and acellular pertussis (DTaP)/H influenza type b/inactive polio vaccination (IPV) (along with oral polio vaccination (OPV)), pneumococcus and hepatitis A in 16-19-month old infants.
Return to the table of contents
Methods
Return to the table of contents
A total of 694 healthy infants and children receiving their regular vaccinations aged between 16 and 19 months attending the well-child unit of the Department of Pediatrics were recruited and randomized for the study between June 2013 and June 2014. Overall, 537 infants were analyzed. These infants were born at term, were of normal birth weight, were otherwise healthy, and were required only routine well-child care.
Permission to perform the study was obtained from the ethics committee of the Dr. Sami Ulus Children and Maternity Training Hospital. Families were approached by a research assistant who explained the nature of the investigation and obtained informed consent.
Sixteen to 19-month-old infants were then randomly assigned to one of three treatment groups: (1) an experimental (75 % sucrose solution), (2) an experimental (25 % sucrose solution), or (3) a control (sterile water solution) group according to the closed envelope technique. The water and sucrose solutions were prepared in advance by a pharmacist. Solutions were drawn from coded bottles and were administered using a syringe. The nurse and parents were blinded to the nature of the solutions used throughout the study. The solutions were placed in coded oral syringes by the pharmacist to ensure that parents, nurses, and investigators were blind to the group assignments. Two minutes prior to the injection, the control and experimental groups received orally 2 mL of either the sterile water or the 25 % sucrose solution or the 75 % sucrose solution.
The infants’ ages and genders were recorded, and participating mothers were asked questions about their educational and socioeconomic levels. The educational attainment was classified as either “no formal schooling: illiterate,” “primary education,” “secondary education,” or “university education.” The socioeconomic level was classified according to monthly household incomes and the official 2013 poverty thresholds of the Turkish Statistical Institute ( http://&www.&tuik.&gov.&tr). Any previous painful experience was assessed by questioning mothers (previous venipuncture, heel prick test, or neonatal circumcision).
The mothers were seated with the infant or child in her arms. The single nurse practitioner used her usual soothing techniques (encouraging parents to cuddle the infant over one shoulder while she employed a distracting, low pitched rattling noise). The use of an infant pacifier or pretreatment with paracetamol was specifically noted. All infants were awake at the time of the procedure. The nurse administered all the test solutions orally. Following the test solution administration, which was given over a period of up to 15 s, OPV was given orally, and intramuscular diphtheria, tetanus, and acellular pertussis (DTaP), intramuscular Haemophilus influenza type b/IPV (pentavalent (DPT+Hib+IPV)), intramuscular pneumococcus, and intramuscular hepatitis A were administered in the deltoid area of the right and left arms at the same time. For infants who are vaccinated at one year of age, pneumococcus vaccination was not administered. Thus, three or two injections were given to all infants, and the entire procedure was videotaped.
The primary study outcome, crying time, was defined as crying from the moment of needle insertion until all crying activity had ceased was recorded by the pediatrician. Acute behavioral pain response was assessed by using the CHEOPS as an objective scale [9, 23, 35]. The scale includes six categories of pain behavior: cry, facial expression, verbal, torso, touch, and legs. The CHEOPS forms were completed by the same pediatrician. A score above 4 indicates pain; the maximum score is 13. The principal investigator responsible for recording the crying time and pain score was not present during the interventions and was blinded to each subject’s treatment.
Return to the table of contents
Statistical analysis
Return to the table of contents
Given the type 1 error (α) of 0.05, the sampling error of 0.05 and power of 90 % sample size was calculated as 383 (n=p . q (Z α+Z β)2/d 2) [21].
SPSS 16.0 for Windows (Chicago, IL, USA) was used for statistical analysis. An analysis of variance (ANOVA) test was used to investigate differences between means of more than two groups, and the chi-square test was used to compare rates of between two or more groups. Binary logistic regressions were used to determine independent factors influencing dependent two-categorical variables. Statistical significance was set at p<0.05 .
Return to the table of contents
Results
Return to the table of contents
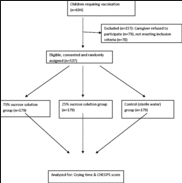
Six hundred ninety-four (n=694) infants were enrolled in this study [20]. One hundred fifty-seven infants were withdrawn after randomization. The remaining 537 infants were randomized to receive 2 mL of a 75 % sucrose solution, a 25 % sucrose solution or sterile water before three or two injections. Figure 1 shows the study flow diagram and the eligible patients.
Table 1 gives demographic and clinical details of how the infants were distributed between the groups. A pacifier was used with only five infants, and paracetamol was administered to eight infants by the parent before immunization. Three children had hemophilia. The administration of test solutions was well tolerated by all infants, and there was no significant difference between the groups in age, weight, sex, previous painful experience, vaccination time, of injection number, pacifier use, and paracetamol administration.
Table 2 gives the crying times and CHEOPS scores recorded in the sucrose treatment groups compared with control groups. Infants receiving 75 % sucrose solution showed significantly reduced total crying times and significantly reduced CHEOPS scores compared with the control and 25 % sucrose solution group (p<0.001). There was a statistically significant difference between the crying times of control and both intervention groups (p<0.001) (Table 2). Both intervention groups (25 vs. 75 % sucrose) were also significantly different from each other (t=8.11, p<0.001). CHEOPS ratings were also significantly different between the control and both intervention groups (p<0.001) (Table 2). Both intervention groups (25 vs. 75 % sucrose) were also significantly different from each other (χ 2=25.08, p<0.001).
In 275 (51 %) infants, pain scores were greater than the cutoff value (CHEOPS score of 4). Binary logistic regression analyses were performed to determine independent factors associated with higher pain scores during vaccinations. CHEOPS scores ≤4 and >4 were used as the dependent variable. The forward-likelihood ratio was used as a method. Age, sex, body weight, maternal education, socioeconomic status, previous pain experience, number of injections, injection time, and interventions were included in the analysis. For categorical variables, the first category was taken as a reference category. In decreasing order of significance, intervention, socioeconomic status, sex, previous pain experience, and body weight were determined as significant independent factors (p<0.05) (Table 3).
Return to the table of contents
Discussion
Return to the table of contents
In Turkey, the routine vaccination schedule includes multiple injections at 16-19 months (DTaP/Haemophilus influenza type B/IPV vaccine, pneumococcus, hepatitis A vaccines, and oral polio vaccine). Before a child reaches 24 months of age in Turkey, he or she should have received 21 injections. The 2013 immunization schedule requires that infants and toddlers receive as many as four injections at a single visit. Parents often report withholding follow-up immunizations from their infants because of their distress [21]. This contributes to reduced community compliance with the recommended schedules. Therefore, it is important to examine interventions to minimize any pain associated during vaccines delivery and to research the evidence for current clinical practice in Turkey.
We showed that the administration of a 75 % sucrose solution before immunization injections reduces infant crying times by nearly 64 % in 16-19-month-old infants. We also performed binary logistic regressions and determined independent factors associated with higher pain scores during vaccinations. The intervention type was determined as the most significant independent factors (p<0.05). Although some studies showed that the effect of sucrose is less pronounced in infants as compared with newborns, some studies reported that sucrose maintains some analgesic action until the prepubertal age [4, 21, 24-26].
The mechanism underlying the analgesic effect of sweet-tasting solutions is considered to involve an orally mediated release of endogenous opioids [6]. This mechanism may work the same way in children as it does in infants older than 1 year of age. Although conflicting results between studies were highlighted, the overall conclusions were that sucrose solutions continued to reduce procedural pain in infants up to 12 months of age, although these effects appeared more modest than in newborn infants [13, 15, 19].
The majority of studies were performed in newborn or younger infants [14, 15, 34]. Allen and Dilli et al. had also studied sucrose solution efficacy in our 16-19-month-old age group. However, they used a 12 % sucrose solution in their studies, which is less concentrated than used in our study [1, 11]. Although, Dilli et al. found a similar profound effect to our study, using a 12 % sucrose solution, on crying time and pain scores during immunizations, they did not find a significant effect. Both studies [1, 11] had potential sources of bias due to their small sample sizes of subgroups of eligible children [16]. Furthermore, data from the Allen et al. study were restricted to measures of crying. Behavioral pain responses can have other relevant features (e.g., heart rate and body movements) [1]. Limiting the response measures to the duration of crying may have missed changes in other behavioral pain-response dimensions.
In our study, the reduced effect of using the 25 % sucrose solution when compared to the 75 % sucrose solution, suggests a dose-related effect of sucrose in older infants. The heightened behavioral pain responses observed in infants receiving 25 % oral sucrose solution reflected greater pain intensity compared with the infants who received 75 % oral sucrose solution. This may be due to reduced sensitivity to sugars as compared to the younger infants who have higher sensitivity to sugars. Our results supported some studies which established that the analgesic effect was dependent on the most concentrated sweet taste. Similar to our study, Ramenghi et al. found that babies receiving the most concentrated sucrose solution in each age group undergoing the same injection cried for a shorter time as compared with the infants receiving the placebo. But they studied 2-4-month old infants [28].
In the present study, current evidence-based distraction techniques as well as parental holding and comforting were considered as standard care and were applied to study groups and the control group [10, 11]. Both the control group and the sucrose groups in our study were securely held in the mother’s arms, while, in some studies, control group infants were placed on an examination table for the injections [1, 30]. The lack of effect of sucrose during immunization reported by Allen et al. may be attributed to the different techniques used to hold the children [1]. The role of parents who held their infants securely during the painful procedure may have resulted in a synergistic effect with sucrose, contributing to the significant reduction in crying and pain scores compared to those infants in the control group [22]. In the study by Reis EC et al., it was not possible to determine whether oral sucrose solution achieved the beneficial effect they observed [30]. The denial of such practices was not thought to be ethical by us. Therefore, we were unable to separate the maternal effect from the sugar effect in the infants we studied.
In our study, we have shown that previous pain experience was a significant independent factor for higher CHEOPS pain scores. Early painful experiences affect children’s future response to analgesia. Weisman et al. found that inadequate analgesia in young children during initial procedures diminished the effects of adequate analgesia during subsequent procedures [37]. The plasticity of the developing brain and the changes that occur in response to painful stimuli also contribute to altered perceptions of pain later in life [12, 17].
Blass et al. concluded that, in the absence of a sucking response, the calming effect of sucrose may be nonspecific; that is, the analgesic effects may be more closely linked to a feature common to water and sucrose rather than a feature specific to sucrose [7, 8]. In our study, the solution was rapidly administered into the infants’ mouth as other studies [1, 4, 21]. The possibility that rapid absorption of sugars through the buccal mucosa may be involved in this process [7, 27]. Some researchers deliberately gave the solution for over 1 min onto the anterior part of the tongue to best stimulate taste senses and sucking actions [29]. One limitation of our study may be in assessing pain response in infants. Recent evidence suggests that multivariable instruments that include physiologic, behavioral, and contextual indicators yield composite pain scores that are more predictive and valid measures of pain in infants [5, 18]. We could not assess any changes in physiological variables such as heart rate and oxygen saturation [28]. But, unlike other published studies, all immunizations were given by the same nurse using her routine methods of soothing for each infant, blinded to the solution they had received [16].
A potential disadvantage when our study results are introduced into practice may be that parents can use too often the intraoral sugars that are highly cariogenic substances, particularly in older infants. However, the volume of 2 mL (less than half a teaspoonful) is comparable in volume and sugar content to commonly administered syrups, including antibiotics and antipyretics. There is minimal risk to infant dentition by the infrequent administration of sucrose in this fashion.
Sucrose, which is inexpensive and is easily administered by individuals without professional training, may be preferred and used during immunization in 16-19-month-old infants. Additional future research can be planned to determine sucrose solution use for pain prevention during other procedures in ambulatory practice sites and hospital settings, in this age group.
Return to the table of contents
References
Return to the table of contents
[1.]Allen KD, White DD, Walburn JN (1996) Sucrose as an analgesic agent for infants during immunization injections. Arch Pediatr Adoles Med 150(3):270-274
[2.]American academy of pediatrics, committee on psychosocial aspects of child and family health. Task Force on Pain in Infants Children and Adolescents (2001) The assessment and management of acute pain in infants, children, and adolescents. Pediatrics 108:793-797
[3.]Anand KJ (2001) International evidence-based group for neonatal pain: consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adoles Med 155:173-180
[4.]Barr RG, Young SN, Wright JH, Cassidy KL, Hendricks L, Bedard Y, Yaremko J, Leduc D, Treherne S (1995) “Sucrose analgesia” and diphtheria-tetanus-pertussis immunizations at 2 and 4 months. J Dev Behav Pediatr 16:220-225
[5.]Bellieni CV, Sisto R, Cordelli DM, Buonocore G (2004) Cry features reflect pain intensity in term newborns: an alarm threshold. Pediatr Res 55(1):142-146
[6.]Blass E, Ciaramitaro V (1994) A new look at some old mechanisms in human newborns. Monogr Soc Res Child Dev 59:1-80
[7.]Blass EM, Hoffmeyer LB (1991) Sucrose as an analgesic for newborn infants. Pediatrics 87:215-218
[8.]Blass EM, Shah A (1995) Pain-reducing properties of sucrose in human newborns. Chem Senses 20:29-35
[9.]Blount RL, Loiselle KA (2009) Behavioral assessment of pediatric pain. Pain Res Manag 14(1):47-52
[10.]Chambers CT, Taddio A, Uman LS, McMurtry CM, HELPinKIDS Team (2009) Psychological interventions for reducing pain and distress during routine childhood immunizations: a systematic review. Clin Ther 31(Suppl 2):S77-S103. doi: 10.&1016/&j.&clinthera.&2009.&07.&023
[11.]Dilli D, Küçük I, Dallar Y (2009) Interventions to reduce pain during vaccination in infancy. J Pediatr 154(3):385-390
[12.]Grunau RV, Holsti L, Peters JW (2006) Long-term consequences of pain in human neonates. Semin Fetal Neonatal Med 11(4):268-275
[13.]Harrison D (2008) Oral sucrose for pain management in the paediatric emergency department; a review. Austral Em Nurs J 11:72-79
[14.]Harrison D, Bueno M, Yamada J, Adams-Webber T, Stevens B (2010) Analgesic effects of sweet tasting solutions in infants: do we have equipoise yet? Pediatrics 126(5):894-902
[15.]Harrison D, Stevens B, Bueno M, Yamada J, Adams-Webber T, Beyene J, Ohlsson A (2010) Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Arch Dis Child 95(6):406-413
[16.]Harrison D, Yamada J, Adams-Webber T, Ohlsson A, Beyene J, Stevens B (2011) Sweet tasting solutions for reduction of needle-related procedural pain in children aged one to 16 years. Cochrane Database Syst Rev 10, CD008408
[17.]Hatfield LA, Gusic ME, Dyer AM, Polomano RC (2008) Analgesic properties of oral sucrose during routine immunizations at 2 and 4 months of age. Pediatrics 121(2):e327-e334
[18.]Johnston CC, Sherrard A, Stevens B, Franck L, Stremler R, Jack A (1999) Do cry features reflect pain intensity in preterm neonates? A preliminary study. Biol Neonate 76(2):120-124
[19.]Kassab M, Foster JP, Foureur M, Fowler J (2012) Sweet-tasting solutions for needle-related procedural pain in infants one month to one year of age. Cochrane Database Syst Rev 12, CD008411. doi: 10.&1002/&14651858.&CD008411.&pub2
[20.]Lachin JM (2008) Sample size evaluation for a multiply matched case-control study using the score test from a conditional logistic (discrete Cox PH) regression model. Stat Med 27(14):2509-2523
[21.]Lewindon PJ, Harkness L, Lewindon N (1998) Randomised controlled trial of sucrose by mouth for the relief of infant crying after immunization. Arch Dis Child 78(5):453-456
[22.]Lewis M (1992) Individual differences in response to stress. Pediatrics 90(3 Pt 2):487-490
[23.]McGrath PJ, Johnson G, Goodman JT (1985) CHEOPS: a behavioral scale for rating postoperative pain in children. In: Fields HL, Dubner R, Cervero F (eds) Advances in pain research and therapy, vol 9. Raven, New York, pp 395-402
[24.]Mennella JA, Pepino MY, Lehmann-Castor SM, Yourshaw LM (2010) Sweet preferences and analgesia during childhood: effects of family history of alcoholism and depression. Addiction 105(4):666-675
[25.]Miller A, Barr G, Young SN (1994) The cold pressor test in children: methodological aspects and the analgesic effect of intraoral sucrose. Pain 56:175-183
[26.]Pepino MY, Mennella JA (2005) Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain 119(1-3):210-218
[27.]Ramenghi LA, Evans DJ, Levene MI (1999) “Sucrose analgesia”: absorptive mechanism or taste perception? Arch Dis Child Fetal Neonatal Ed 80(2):F146-F147
[28.]Ramenghi LA, Webb AV, Shevlin PM, Green M, Evans DJ, Levene MI (2002) Intra-oral administration of sweet-tasting substances and infants’ crying response to immunization: a randomized, placebo-controlled trial. Biol Neonate 81:163-169
[29.]Ramenghi LA, Wood CM, Grifith GC, Lvene MI (1996) Reduction of pain response in premature infants using intraoral sucrose. Arch Dis Child Fetal Neonatal Ed 74:126-128
[30.]Reis EC, Roth EK, Syphan JL, Tarbell SE, Holubkov R (2003) Effective pain reduction for multiple immunization injections in young infants. Arch Pediatr Adolesc Med 157(11):1115-1120
[31.]Royal Australasian College of Physicians (2005) Guideline statement: management of procedure-related pain in neonates. Sydney: Paediatrics & Child Health Division, The Royal Australasian College of Physicians
[32.]Schechter NL, Zempsky WT, Cohen LL, McGrath PJ, McMurtry CM, Bright NS (2007) Pain reduction during pediatric immunizations: evidence-based review and recommendations. Pediatrics 119:e1184-e1198
[33.]Shah V, Taddio A, Rieder MJ (2009) Effectiveness and tolerability of pharmacologic and combined interventions for reducing injection pain during routine childhood immunizations: systematic review and meta-analyses. Clin Ther 31(Suppl 2):S104-S151
[34.]Stevens B, Yamada J, Ohlsson A (2010) Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews, Issue 1. doi: 10.&1002/&14651858.&CD001069.&pub3
[35.]Suraseranivongse S, Santawat U, Kraiprasit K, Petcharatana S, Prakkamodom S, Muntraporn N (2001) Cross-validation of composite pain scale for preschool children within 24 hours of surgery. Br J Anaesth 87:400-405
[36.]Taddio A, Manley J, Potash IM, Sgro M, Shah V (2007) Routine immunization practices: use of topical anesthetics and oral analgesics. Pediatrics 120:e637-e643
[37.]Weisman SJ, Bernstein B, Schechter NL (1998) Consequences of inadequate analgesia during painful procedures in children. Arch Pediatr Adolesc Med 152(2):147-149
Return to the table of contents
European Journal of Pediatrics 2014; aop: 10.1007/s00431-014-2358-7
Copyright Springer-Verlag 2014 powered by GNM Healthcare Consulting Group, LLC
© 2014 GNM Healthcare Consulting Group, LLC.